
图1 余甘子酚类成分(1~7)化学结构
Fig.1 Chemical structures of the phenolic compounds isolated from Phyllanthus emblica L
摘要:研究余甘子甲醇提取物降糖活性部位中的酚类化学成分及其抑制 α-葡萄糖苷酶活性。采用甲醇浸泡提取余甘子,D101大孔树脂柱粗分离得到三个部位,降糖活性测试表明30%乙醇洗脱部位(PEA)为活性部位,后续采用Sephadex LH-20,ODS和制备HPLC等柱色谱技术对其进行分离纯化,根据化合物理化性质、NMR和HR-ESI-MS数据并参考相关文献鉴定化合物结构,采用体外方法测定化合物抑制α-葡萄糖苷酶活性。从PEA部位中分离并鉴定了7个酚类化合物,分别为:没食子酸(1),没食子酸甲酯(2),1-O-没食子酰基-β-D-葡萄糖苷(3),1,6-二-O-没食子酰基-β-D-葡萄糖苷(4),柯里拉京(5),粘酸-1,4-内酯-2-O-没食子酸酯(6),粘酸-2-O-没食子酸酯(7)。除化合物2外所有化合物均具有一定的抑制α-葡萄糖苷酶活性。没食子酸衍生物是余甘子抑制α-葡萄糖苷酶活性成分。
关键词:余甘子;化学成分;酚类成分;α-葡萄糖苷酶抑制活性
余甘子(Phmllanthi Fructus)是大戟科(Euphorbiaccae)叶下珠属(Phyllanthus)植物余甘子(Phyllanthus emblica. L)的成熟果实,广泛分布于我国东南沿海和西南等省区,其药用价值广泛,收载于多版《中华人民共和国药典》,1998年被卫生部列为“既是食品又是药品”目录。它具有清热凉血,生津止咳,消食健胃之功效,可用于治疗血热血淤、消化不良、腹胀、咳嗽、喉痛及口干等证候。现代化学成分研究表明,余甘子主要成分为多酚类化合物[1,2],另外还有黄酮、甾醇、生物碱、多糖、维生素、氨基酸以及微量元素等[3],具有降血糖、护肝和抗肝炎病毒[4]、抗菌、抗病毒、抗氧化[5]、清除自由基、抗肿瘤[6]、抗炎镇痛、抗动脉粥样硬化和抗疲劳、抗缺氧等药理活性,在糖尿病、肝病、癌症、心血管疾病及贫血等疾病的治疗中发挥重要的保健作用[7]。
α-葡萄糖苷酶是存在于小肠绒毛膜刷状缘上的催化淀粉、蔗糖和麦芽糖水解为葡萄糖和果糖的一组酶系,对调节体内碳水化合物的代谢具有重要的作用;α-葡萄糖苷酶抑制剂可以竞争性抑制α-葡萄糖苷酶的活性,从而减缓淀粉和蔗糖等向葡萄糖的转化,进而控制餐后血糖的水平[8]。以α-葡萄糖苷酶为靶点筛选植物中具有调节血糖作用的活性物质引起了广泛的关注,是研究和开发治疗II型糖尿病药物和保健食品的重要靶点[9]。
王锐等[10]研究了余甘子多酚抑制 α-葡萄糖苷酶及抗氧化活性,但是并没有系统分离鉴定余甘子的多酚成分;已有研究报道余甘子水提取物可以降低四氧嘧啶和链脲佐菌素(STZ)诱导的糖尿病鼠模型血糖水平[11~13],但其发挥降血糖作用的活性物质及其作用机制还不明确。前期我们研究发现余甘子甲醇提取物可以显著升高GLUT-2和PPARγ mRNA的表达和PPRE报告基因的活性,抑制脂多糖(LPS)诱导的炎症反应,降低NF-κB报告基因的活性,且随着浓度的升高其抑制作用增强。大孔树脂柱30%乙醇洗脱物为其有效活性部位,1H-NMR分析也表明没食子酸及其糖苷类衍生物为其主要成分[14]。为了进一步研究余甘子发挥降血糖作用的活性物质,本研究继续研究余甘子大孔树脂柱 30%乙醇洗脱物(PEA)的化学成分,以期发现其发挥降血糖作用的活性物质基础。
柱色谱填料:D101大孔树脂(沧州宝恩吸附材料科技有限公司),葡聚糖凝胶 Sephadex LH-20(GE公司),ODS (YMC);所有分析纯溶剂(石油醚、氯仿、乙酸乙酯、正丁醇、异丙醇和甲醇等)。α-葡萄糖苷酶(Yeast,EC 3.2.1.20)、4-硝基苯-α-D-吡喃葡萄糖苷和阿卡波糖(美国Sigma公司)。余甘子2015年10月购自江西樟树药材大市场,凭证标本(YG-201510)存放于江西省果蔬保鲜与无损检测重点实验室。
核磁共振光谱利用瓦里安 500 MHz型核磁共振仪测定(TMS内标),HR-ESI-MS由(Applied Biosystems MDS)质谱仪测定,分析和半制备高效液相色谱为Hitachi Elite LaChrom系统包括L2130泵,L-2200自动进样器,L-2455二极管阵列检测器和菲罗门 Luna C18色谱柱(250 mm×10 mm,5 μm),SpectraMax M2多功能读板机(Molecular Devices,USA)。
1.3.1 提取与分离
1 kg余甘子粉末用5 L甲醇室温浸提3次(每次5 d),合并滤液减压浓缩,得总浸膏110 g。取100 g浸膏溶解于1.5 L蒸馏水中,采用D101大孔树脂柱进行分离,依次用3 L的水、30%乙醇、50%乙醇和90%乙醇洗脱,收集醇洗脱部位(PEA、PEB和PEC),进行活性测试及后续柱层析分离[14]。PEA经C18中压色谱(MPLC)分离(4 cm×30 cm),甲醇:水梯度洗脱(1:9→6:4,V/V),得到 4 流份(PEA1~PEA4)。PEA1 经Sephadex LH-20凝胶柱色谱分离(甲醇洗脱)后经半制备液相分离(流动相为 10%甲醇等度,流速 3.2 mL/min)得到化合物6(8.8 mg)和7(9.6 mg),PEA2经Sephadex LH-20凝胶柱色谱分离(甲醇洗脱)后经半制备液相分离(流动相为 12%甲醇等度,流速 3.2 mL/min)得到化合物1 (408 mg)和3 (56 mg),PEA3经Sephadex LH-20凝胶柱色谱分离(甲醇洗脱)后经半制备液相分离(流动相为18%甲醇至35%甲醇,流速3.2 mL/min)得到化合物2 (12.8 mg),4 (11.6 mg)和5 (13.5 mg)。
1.3.2 α-葡萄糖苷酶抑制活性的测定
参照文献的方法[15],分别于96孔板中加入50 μL不同浓度的溶于DMSO的被测试样品,50 μL磷酸盐缓冲液和100 μL 1 U/mL的α-葡萄糖苷酶溶液,每个样品3个复孔,37 ℃恒温孵育10 min后加入5 mM pNPG溶液50 μL,立即于405 nm波长下测定其OD值(S0min),反应5 min后相同波长条件下测定取OD值(S5min),加入相同体积的缓冲液作为空白对照,阿卡波糖为阳性对照。按照下面的公式计算抑制率:
抑制率%=100×[(C5-C0)-(S5-S0)]/(C5-C0)
C0为加入缓冲液代替样品的空白对照0 min时的OD值,C5空白对照5 min时的OD值,S0为加入测试样品0 min时的OD值,S5为加入测试样品5 min时的OD值。
1.3.3 数据统计分析
NMR数据采用MestReNova 6.1.0软件分析,α-葡萄糖苷酶抑制活性采用Excel 2003进行数据分析并作图。
通过分析NMR和HR-ESI-MS数据,并参考已知化合物波谱数据的相关文献鉴定化合物1~7(结构见图1)的结构。

图1 余甘子酚类成分(1~7)化学结构
Fig.1 Chemical structures of the phenolic compounds isolated from Phyllanthus emblica L
没食子酸 (1)白色粉末,UV (MeOH) λmax:276 nm;(-) HR-ESI-MS, m/z 169.0110 [M-H]-,可知化合物1的分子式为C7H6O5;1H-NMR (500 MHz, CD3OD, δ, ppm,J/Hz): 7.06 (2H, s, H-galloyl-2, 6)为芳环上 2 个对称的质子信号,结合13C-NMR (125 MHz, CD3OD): 108.9(C-2, 6), 120.5 (C-1), 138.1 (C-4), 145.0 (C-3, 5), 168.9(C-7),化合物1的NMR数据与文献[16]报道基本一致,确定化合物1为没食子酸。
没食子酸甲酯(2)白色粉末,UV (MeOH) λmax: 276 nm;(-) HR-ESI-MS, m/z 183.0099 [M-H]- (确定其分子式为 C8H7O5)可知化合物 2的分子式为 C8H8O5;1H-NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 6.94 (2H, s,H-galloyl-2, 6)为芳环上2个对称的质子信号,3.71 (3H,s, OCH3)为甲氧基信号,结合 13C-NMR (125 MHz,CD3OD): 108.6 (C-2, 6), 120.0 (C-1), 138.3 (C-4), 145.1(C-3, 5), 167.6 (C-7), 50.8 (COOCH3),化合物2的NMR数据和化合物1类似,仅多了一个甲氧基信号,化合物2的NMR数据与文献[16]报道基本一致,确定化合物2为没食子酸甲酯。
1-O-没食子酰基-β-D-葡萄糖苷(3):白色粉末,(-)HR-ESI-MS, m/z: 331.1822 [M-H]-。1H-NMR (500 MHz,CD3OD, δ, ppm, J/Hz): 7.12 (2H, s, H-Galloyl-2, 6), 5.65(1H, d, J = 8. 4 Hz, H-Glc-1'), 3.85 (1H, dd, J = 1.7, 12.0 Hz, H-Glc-6'eq), 3.69 (1H, dd, J =4.6, 12.1 Hz, H-6'ax),3.40-3.48 (4H, m, H-Glc-2', 3', 4', 5'); 13C-NMR (125 MHz, CD3OD): 119.3 (C-1), 109.1 (C-2, 6), 145.1 (C-3,5), 138.9 (C-4), 165.6 (C-7), 94.5 (C-1'), 72.7 (C-2'), 77.4(C-3'), 69.7 (C-4'), 76.8 (C-5'), 60.9 (C-6')。NMR数据表明化合物3结构中含有一个没食子酰基和一个己碳糖,以上数据与文献[17]报道对照基本一致,故鉴定化合物3为1-O-没食子酰基-β-D-葡萄糖。
1,6-二-O-没食子酰基-β-D-葡萄糖苷(4)白色粉末,(-) HR-ESI-MS, m/z: 483.2469 [M-H]-。1H-NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 7.04 (2H, s, H-Galloyl-2,6), 6.98 (2H, s, H-Galloyl-2', 6'), 5.59 (1H, d, J = 7.2 Hz,H-Glc-1''), 4.46 (1H, dd, J = 12.1, 1.8 Hz, H-Glc-6''eq),4.30 (1H, dd, J = 12.1, 5.0 Hz, H-Glc-6''ax), 3.62 (1H, m,H-Glc-5''), 3.43-3.41 (3H, m, H-Glc-2'', 3'', 4''); 13C-NMR(125 MHz, CD3OD): 166.9 (C-7), 165.6 (C-7'),145.1(C-3, 5), 145.0 (C-3', 5'), 139.0 (C-4), 138.4 (C-4'),119.9 (C-1), 119.2 (C-1'), 109.2(C-2, 6), 108.8 (C-2', 6'),94.5 (C-1''), 76.6 (C-3''), 75.0 (C-5''), 72.7 (C-2''), 69.8(C-4''), 63.0 (C-6'')。NMR数据表明化合物4比化合物3多一个没食子酰基,根据葡萄糖H-6向低场位移,表明H-6连有一个没食子酰基。以上数据与文献[18]报道一致, 故鉴定化合物4为1,6-二-O-没食子酸-β-D-葡萄糖苷。

图2 化合物5结构(a)关键的HMBC相关谱(H→C)(b)
Fig.2 The key HMBC correlation spectrum (H→C) (b) of compound 5 structure (a)
柯里拉京(5)白色粉末,(-) HR-ESI-MS, m/z:633.2960 [M-H]-。1H-NMR (500 MHz, CD3OD, δ, ppm,J/Hz): 7.05 (2H, s, H-Galloyl-2', 6') 为没食子酰基,6.69(1H, s, H-Galloyl-5'')和 6.66 (1H, s, H-Galloyl-5''')为HHPD信号,6.36 (1H, d, J =2.1, H-Glc-1)为葡萄糖端基H信号,4.96 (2H, t, J=11.0 Hz, H-Glc-3, 5), 4.52 (1H,t, J=8.5 Hz, H-Glc-4), 4.46 (1H, dd, J=1.8, 11.0 Hz,H-Glc-6eq), 4.15 (1H, dd, J=8.5, 11.0 Hz, H-Glc-6ax),3.98 (1H, m, H-Glc-2);13C-NMR (125 MHz, CD3OD):61.1 (C-4), 63.6 (C-6), 68.1 (C-2), 70.3 (C-3), 74.8 (C-5),93.6 (C-1), 106.9 (C-2''), 108.7 (C-2'''), 109.5 (C-2', 6'),115.3(C-6''), 115.8(C-6'''), 119.2 (C-1'), 124.0 (C-1''),124.0 (C-1'''), 136.3(C-4''), 136.7 (C-4'''), 139.0 (C-4'),143.8 (C-3''), 143.9 (C-3'''), 144.2 (C-5''), 144.6 (C-5'''),145.0 (C-3', 5'), 165.3 (C-7'), 167.1 (C-7''), 168.7 (C-7''')。通过分析1H-1H COSY谱确定了葡萄糖H的化学位移,进一步分析HMBC谱(关键HMBC相关见图2),确定没食子酰基和HHPD的连接位置,葡萄糖端基H信号δH 6.36和δC165.3 (C-7')相关,没食子酰基δH 7.05和δC165.3 (C-7')相关,说明没食子酰基连接在葡萄糖的端基位;HHPD连接在葡萄糖H-3和H-6位置。以上数据与文献[19]报道也一致,故鉴定化合物5为柯里拉京。
粘酸-1,4-内酯-2-O-没食子酸酯(6):白色粉末,ESI-MS, m/z: 343.1510 [M-H]-。1H-NMR (500 MHz,CD3OD, δ, ppm, J/Hz): 7.12 (2H, s, H-Galloyl-2', 6'),5.68 (1H, t, J = 7.2 Hz, H-2), 4.79 (2H, d, J = 7.6 Hz, H-3,4), 4.48 (1H, dd, J =1.8 Hz, H-5); 13C-NMR (125 MHz,CD3OD): 173.1 (C-6), 172.5 (C-1), 166.1 (C-7'), 145.2(C-3', C-5'), 139.0 (C-4'), 118.8 (C-1'), 109.0 (C-2', 6'),80.1 (C-4), 75.3 (C-2), 72.0 (C-3), 68.7 (C-5)。以上数据与文献[20]报道对照基本一致,故鉴定化合物6为粘酸-1,4-内酯-2-O-没食子酸酯。
粘酸-2-O-没食子酸酯(7):白色粉末, ESI-MS, m/z:361.1700 [M-H]-。1H-NMR (500 MHz, CD3OD, δ, ppm,J/Hz): 7.19 (2H, s, H-Galloyl-2', 6'), 5.49 (1H, d, J = 1.6Hz, H-2), 4.51 (1H, s, H-5), 4.37 (1H, dd, J =1.6, 9.9 Hz,H-3) , 4.07 (1H, d, J =10.0 Hz, H-4); 13C-NMR (125 MHz, CD3OD): 171.1 (C-1, 6), 166.4 (C-7'), 145.0 (C-3',C-5'), 138.6 (C-4'), 119.7 (C-1'), 109.0 (C-2', 6'), 72.5(C-2), 71.4 (C-4, 5), 70.1 (C-3)。以上数据与文献[20]报道对照基本一致,故鉴定化合物7为粘酸-2-O-没食子酸酯。
余甘子酚类单体化合物(1~7,结构见图1)抑制α-葡萄糖苷酶活性结果见图3,在测定的最大浓度2000 μM时除化合物2外,其他化合物α-葡萄糖苷酶抑制率均高于50%,表明除化合物2外所有化合物均具有一定的抑制 α-葡萄糖苷酶活性,其 IC50范围为286~1680 μM。
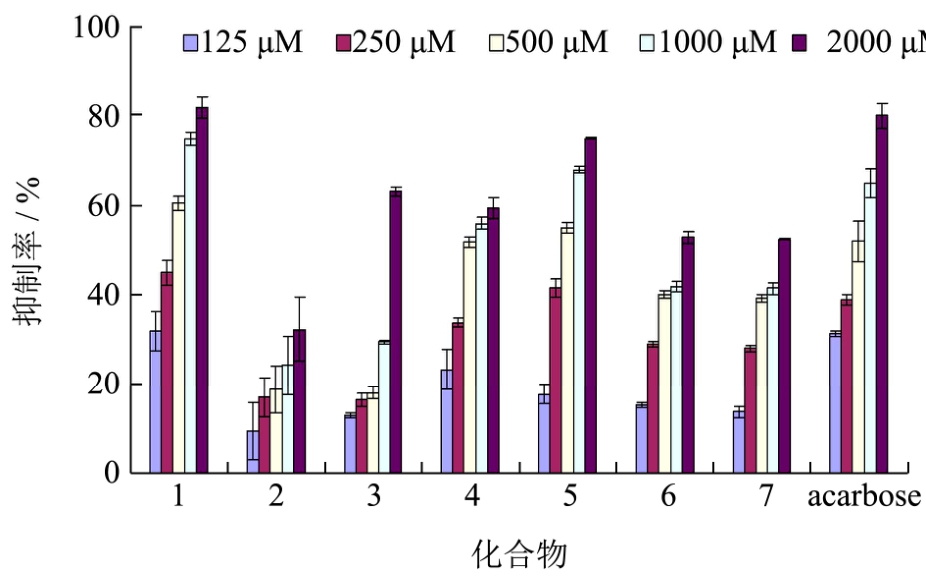
图3 余甘子酚类单体化合物抑制α-葡萄糖苷酶活性
Fig.3 The phenolic monomer compounds isolated from Phyllanthus emblica L inhibited α-glucosidase activity
化合物1没食子酸抑制α-葡萄糖苷酶活性最强,和阳性对照阿卡波糖效果相当;张海凤等[21]也研究了没食子酸对α-葡萄糖苷酶的抑制作用及其机制,发现采用酶-抑制剂模型和 Caco-2细胞模型没食子酸均有效果且为竞争型抑制。化合物2没食子酸甲酯活性最差,表明甲酯化降低了没食子酸抑制α-葡萄糖苷酶活性。化合物4 (1,6-二-O-没食子酰基-β-D-葡萄糖苷)和5 (柯里拉京)活性比没食子酸和阿卡波糖稍弱,IC50为500 μM左右,说明糖基化也降低了没食子酸的抑制α-葡萄糖苷酶活性,有研究[22]表明黄酮类化合物B环的C3-OH,以及A环的C6和C8为糖基化会降低黄酮抑制α-葡萄糖苷酶活性,这与我们的研究结果类似。化合物 3 (1-O-没食子酰基-β-D-葡萄糖苷)、6(粘酸-1,4-内酯-2-O-没食子酸酯)和 7(粘酸-2-O-没食子酸酯)的抑制α-葡萄糖苷酶IC50为1500 μM左右,进一步说明酯化和糖基化降低了没食子酸的活性;但是结构中没食子酰基的数量有助于提高化合物抑制α-葡萄糖苷酶活性[21]。
多酚类化合物是余甘子植物的特征性成分,从余甘子中分离鉴定的多酚类成分主要为没食子酸类衍生物,此外还有黄酮类等化合物[1],这与我们的研究结果一致。从余甘子甲醇提取物的降血糖活性部位中分离鉴定了7个没食子酸衍生的酚类化合物,他们分别为没食子酸(1),没食子酸甲酯(2),1-O-没食子酰基-β-D-葡萄糖苷(3),1,6-二-O-没食子酰基-β-D-葡萄糖苷(4),柯里拉京(5),粘酸-1,4-内酯-2-O-没食子酸酯(6),粘酸-2-O-没食子酸酯(7)。除化合物 2外所有化合物均具有一定的抑制α-葡萄糖苷酶活性。没食子酸衍生物是余甘子抑制α-葡萄糖苷酶的活性成分,没食子酸衍生物是余甘子发挥降血糖活性的主要成分,这与我们前期的研究结果一致[14]。没食子酰化多酚类化合物是余甘子的主要成分,文献报道它们都具有很强的抗氧化、清除自由基和抑制 α-葡萄糖苷酶的活性[21]。本试验结果可为余甘子进一步开发为降血糖保健品提供一定的物质基础。
参考文献
[1] Liu X, Cui C, Zhao M, et al. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities [J]. Food Chemistry, 2008, 109(4):909-915
[2] YANG B, LIU P. Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica [J].Journal of Agricultural and Food Chemistry, 2014, 62(3):529-541
[3] 刘延泽,李海霞,许利嘉,等.药食兼用余甘子的现代研究概述及应用前景分析[J].中草药,2013,44(12):1700-1706 LIU Yan-ze, LI Hai-xia, XU Li-jia, et al. Modern research overview and application prospect analysis on Phyllanthus emblica both as diet and medicine [J]. Chinese Traditional and Herbal Drugs, 2013, 44(12): 1700-1706
[4] Lu C C, Yang S H, Hsia S M, et al. Inhibitory effects of Phyllanthus emblica L. on hepatic steatosis and liver fibrosis in vitro [J]. Journal of Functional Foods, 2016, 20: 20-30
[5] Liu X, Zhao M, Wang J, et al. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.)from six regions in China [J]. Journal of Food Composition and Analysis, 2008, 21(3): 219-228
[6] Ngamkitidechakul C, Jaijoy K, Hansakul P, et al. Antitumour effects of Phyllanthus emblica L.: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells [J]. Phytotherapy Research, 2010, 24(9): 1405-1413
[7] Calixto J B, Santos A R S, Filho V C, et al. A review of the plants of the genus Phyllanthus: their chemistry,pharmacology, and therapeutic potential [J]. Medicinal Research Reviews, 1998, 18(4): 225-258
[8] Kim K T, Rioux L E, Turgeon S L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum [J]. Phytochemistry, 2014, 98: 27-33
[9] Rengasamy K R R, Aderogba M A, Amoo S O, et al.Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss [J]. Food Chemistry,2013, 141(2): 1412-1415
[10] 王锐.余甘子多酚α-葡萄糖苷酶抑制及抗氧化作用[J].食品研究与开发,2017,38(11):13-16 WANG Rui. Inhibition effect on α-Glucosidase and antioxidant activity for polyphenol extracts from Phyllanthus emblica L [J]. Food Research and Development, 2017, 38(11):13-16
[11] 韩刚,原海忠,董月,等.余甘子提取物对糖尿病小鼠血糖的影响[J].食品科学,2009,30(9):210-212 HAN Gang, YUAN Hai-zhong, DONG Yue, et al. Blood glucose-reducing effects of Phmllanthi fructus fruit extracts on diabetic mice [J]. Food Science, 2009, 30(9): 210-212
[12] Mani K, Sankaran M, Kandan K, et al. Antidiabetic and antihyperlipidemic properties of Phyllanthus emblica Linn.(Euphorbiaceae) on streptozotocin induced diabetic rats[J]. Pakistan Journal of Nutrition, 2010, 9(1): 43-51
[13] 康文娟,张广梅,赵协慧,等.不同溶剂余甘子提取物的降血糖作用研究[J].安徽农业科学,2011,39(30):18545-18547 KANG Wen-juan, ZHANG Guang-mei, ZHAO Xie-hui, et al.Hypoglycemic effects of different solvent extracts from Phmllanthi fructus [J]. Journal of Anhui Agricultural Sciences,2011, 39(30): 18545-18547
[14] 李明玺,黄卫锋,姚亮亮,等.余甘子提取物降血糖活性及其主要成分研究[J].现代食品科技,2017,33(9): 96-101 LI Ming-xi, HUANG Wei-feng, YAO Liang-liang, et al.Hpyerglycemic activity of Phyllanthus emblica L and its main composition analysis [J]. Modern Food Science and Technology, 2017, 33(9): 96-101
[15] Wan C, Yuan T, Cirello A L, et al. Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush blueberry flowers [J]. Food Chemistry, 2012, 135(3):1929-1937
[16] 万春鹏,周寿然.红槭树树枝化学成分及抗氧化活性研究[J].林产化学与工业,2013,33(5):93-96 WAN Chun-peng, ZHOU Shou-ran. Chemical constituents of Acer rubrum L and their antioxidant activities [J]. Chemistry and Industry of Forest Products, 2013, 33(5): 93-96
[17] 舒希凯,段文娟,刘伟,等.芍药花化学成分研究[J].中药材,2014,37(1):66-69 SHU Xi-kai, DUAN Wen-juan, LIU Wei, et al. Chemical constituents from flowers of Paeonia lactiflora [J]. Journal of Chinese Medicinal Materials, 2014, 37(1): 66-69
[18] 王景华,李明慧,王亚琳,等.槐种子化学成分研究(Ⅱ)[J].中草药,2002,33(7):586-588 WANG Jing-hua, LI Ming-hui, WANG Ya-lin, et al. Studies on chemical constituents from seeds of Sophora japonica (Ⅱ)[J]. Chinese Traditional and Herbal Drugs, 2002, 33(7):586-588
[19] 朱红霖,韦万兴,周敏,等.珠子草化学成分的研究[J].天然产物研究与开发,2011,23(3):401-403 ZHU Hong-lin, WEI Wan-xing, ZHOU Min, et al. Chemical constituents of Phyllanthus niruri L [J]. Nat Prod Res Dev,2011, 23(3): 401-403
[20] Zhang Y J, Tanaka T, Yang C R, et al. New phenolic constituents from the fruit juice of Phyllanthus emblica [J].Chemical & Pharmaceutical Bulletin, 2001, 49(5): 537-540
[21] 张海凤,董亚琳,张琰.没食子酸对 α-葡萄糖苷酶的抑制作用及其降糖机制研究[J].中国药业,2011,20(21):8-10 ZHANG Hai-feng, DONG Ya-lin, ZHANG Yan. The research of gallic acid’s α-glycosidase inhibition and the hypoglycemic mechanism [J]. China Pharmaceuticals, 2011,20(21): 8-10
[22] Li H, Song F, Xing J, et al. Screening and structural characterization of α-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MS n and SORI-CID FTICR MS [J]. Journal of the American Society for Mass Spectrometry, 2009, 20(8): 1496-1503
[23] Wan C, Yuan T, Li L, et al. Maplexins, new α-glucosidase inhibitors from red maple (Acer rubrum) stems [J].Bioorganic & Medicinal Chemistry Letters, 2012, 22(1):597-600
Structural Identification of Phenolic Constituents from Phyllanthus emblica L. and Their Inhibitory Activity on α-glucosidase
Abstract: The phenolic constituents in the hypoglycemic active fraction of the methanol extract of Phyllanthus emblica L and their inhibitory activity on α-glucosidase were studied. Using methanol soaking extract Phyllanthus emblica L, the methanol extract (PE) was chromatographed on a D101 macroporous resin column to obtain three fractions (PEA-PEC). Hypoglycemic activity test showed that 30%ethanol elution part (PEA) was the active fraction. PEA was further isolated and purified by column chromatography of Sephadex LH-20, ODS and semi-preparative HPLC columns. The structures of the isolated compounds were identified according to the physicochemical properties,1H-NMR, 13C-NMR and high resolution mass spectral analyses as well as relevant references. The compounds were tested for their in vitro inhibitory activity on α-glucosidase. Seven phenolic compounds were isolated from PEA fraction and identified as gallic acid (1), methyl gallate(2), 1-O-galloyl-β-D-glucose (3), 1,6-di-O-galloyl-β-D-glucose (4), corilagin (5), mucic acid-1,4-Lactone-2-O-gallate (6), mucic acid-2-O-gallate (7). All compounds except compound 2 showed good inhibitory activity on α-glucosidase. Gallic acid derivatives were the active constituents that P. emblica L. inhibited the activity of α-glucosidase.
Key words: Phyllanthus emblica L., chemical constituents; phenolic constituents; inhibitory activity of α-glucosidase
文章篇号:1673-9078(2017)12-50-55
DOI: 10.13982/j.mfst.1673-9078.2017.12.008
收稿日期:2017-07-23
基金项目:新疆维吾尔自治区教育厅普通高校重点实验室资助项目(2017YSHXZD01);国家自然科学基金青年科学基金项目(31500286)